Pressure of Hydrogen Gas in Mmhg
The hydrogen gas created inside a chemical reaction is collected over water at 30. When a gas is produced in a chemical reaction it can be collected by water displacement.
Solved Find Pressure Of Hydrogen Gas Mmhg Pressure Of Chegg Com
The barometric pressure at the time was 7425 mm Hg.

. The pressure at collection time was 77050 mm Hg. Pressure of hydrogen gas Total. What is the temperature at standard pressure.
The vapour pressure of water at this temperature is 1910 mm Hg. What is the partial pressure of oxygen at mean sea level. 23 2 P O 2.
Atmospheric pressure 765 mmHg. COg2H2gCH3OHg A 165 L reaction vessel initially at 305 K contains carbon monoxide gas at a partial pressure of 232 mmHg and hydrogen gas at a partial pressure of 374 mmHg Identify the limiting reactant and determine the theoretical yeild of methonal in. What is the pressure of hydrogen gas if the atmospheric pressure is 754 mmHg and the water temperature is 23 degrees C.
The hydrogen gas was collected over the water. Some hydrogen gas is collected over water at 200C. What may be the partial pressure from the hydrogen gas collected in this manner.
Convert 975 mmHg to atmospheres show work Formula. Hydrogen was is collected over water with a total pressure of 7250 mmHg at 185 C. 760 mmHg 5934mmHg 707mmHg 031mmHg P O2 P O2 15922 mmHg.
P O 2 23 2. R 0082057 L atmmol K. 975 mmHg is equal to 128289474 atm.
1 mm Hg 136 mm H2O. X o x y g e n P o x y g e n P t o t a l. According to the Daltons law of partial pressure the total pressure of a gas mixture is the sum of all the partial pressure of the individual gases present in the mixture of gases.
The other gases besides oxygen total 031 mm Hg. This is all abstract but you can bring an actual problem to the table. Boiling Point - saturation pressure 147 psia and 760 mm Hg - o F o K-423 204.
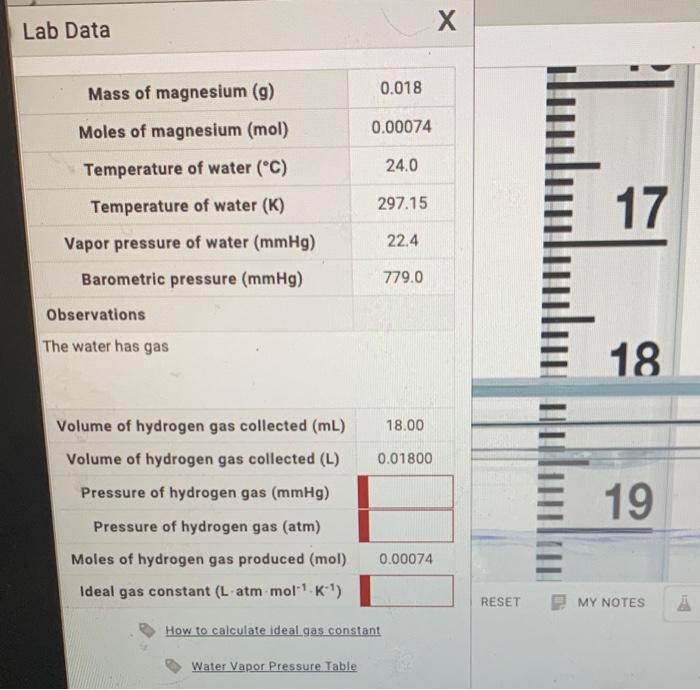
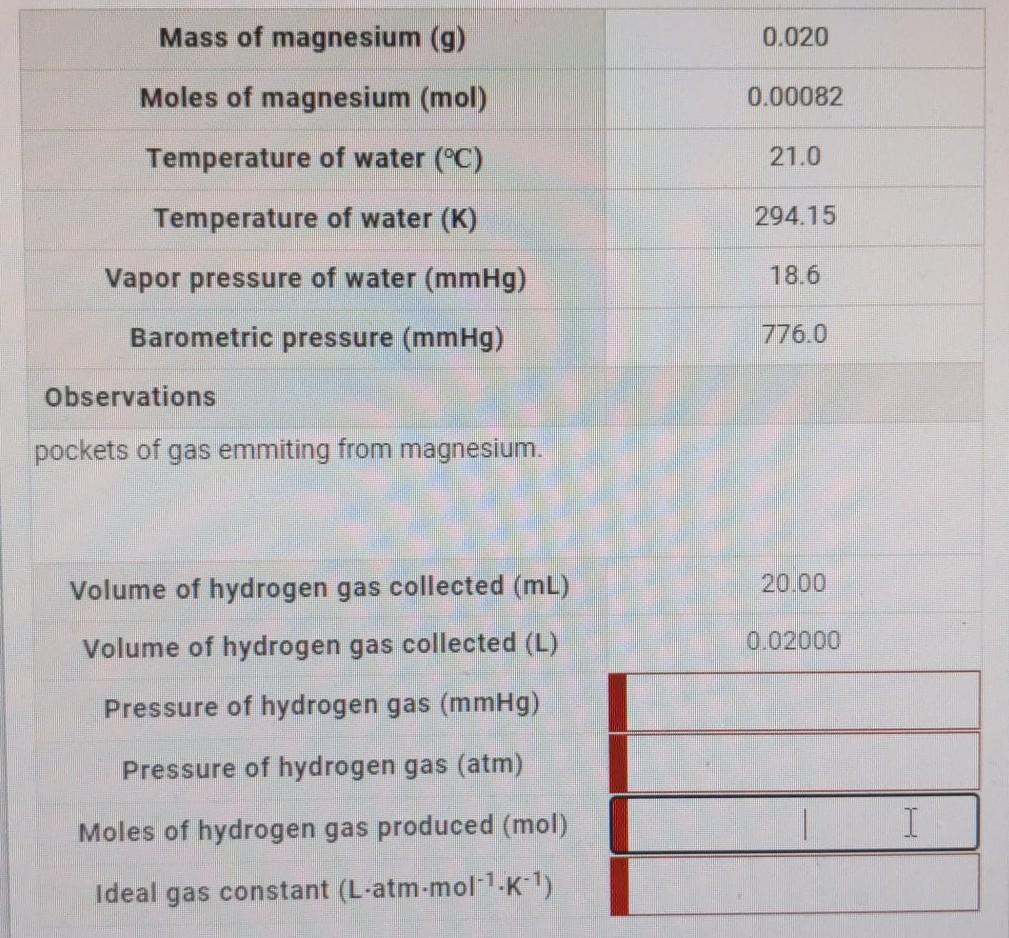
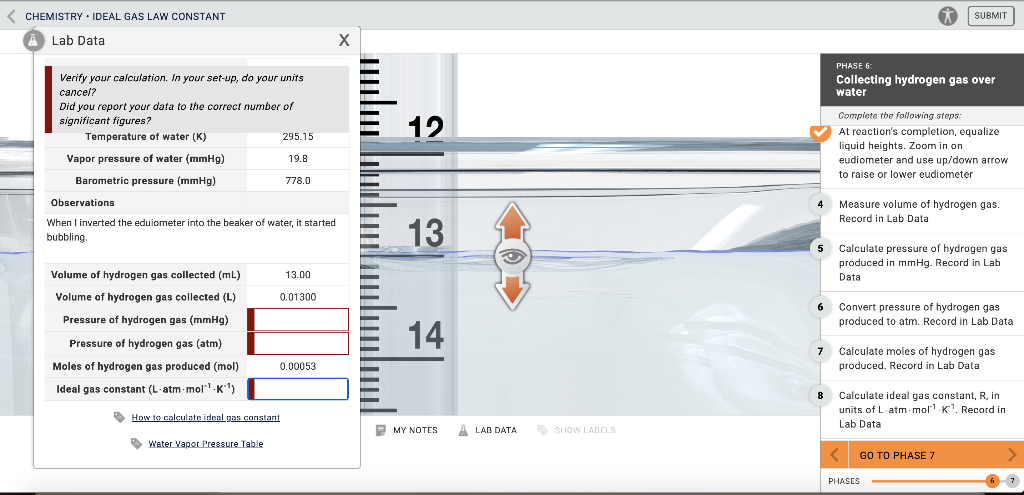
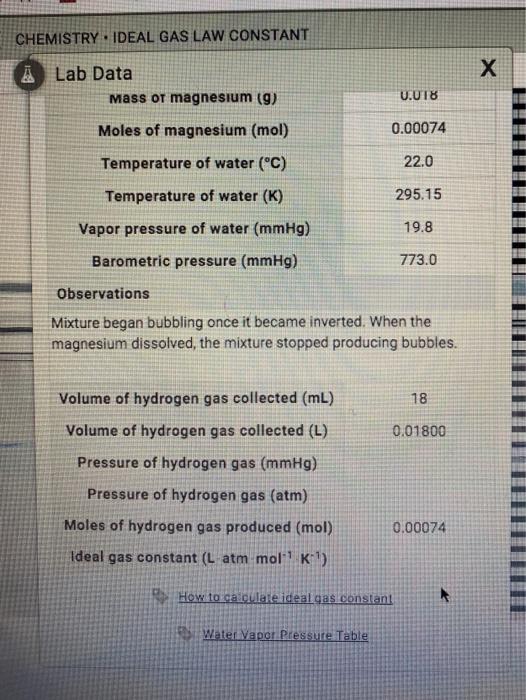
CHEMISTRY - IDEAL GAS LAW CONSTANT INTRODUCTION LABORATORY SIMULATION À Lab Data Mass of magnesium g 0020 Moles of magnesium mol 000082 Temperature of water C 210 Temperature of water K 29415 Vapor pressure of water mmHg 186 Barometric pressure mmHg 7760 Observations pockets of gas being. Total pressure pressure of hydrogen gas vapour pressure of water. What is the pressure of hydrogen gas if the atmospheric pressure is 754 mmHg and the water temperature is 23 degrees C.
6335 mmHg is the pressure of hydrogen gas formed in mmHg. A754 b724 c775 d733 Question Transcribed Image Text. What is collecting gas over water.
Explanation As per Daltons law of partial pressures total pressure is equal to. 17 rows What is the pressure of hydrogen gas in atm. The eudiometer contains 000206 g of hydrogen 00811 g of oxygen and 00151 g of water vapor.
If the gas mixture contains 206 grams of xenon how many grams of hydrogen are present. Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction. Answer 7600 mmHg Daltons Law.
Hydrogen is easily ignited. It is useful to remember that 760 mmHg1000 atm1013 kPa The pressure of a gas in a container is often measured relative to atmospheric pressure using a manometer. A mixture of xenon and hydrogen gases at a total pressure of 965 mm Hg contains xenon at a partial pressure of 313 mm Hg.
What is the partial pressure of the hydrogen gas collected in this way. The partial pressure of hydrogen gas is 7089 mmHg. A sample of hydrogen gas occupies a volume of 856.
What is the pressure of dry hydrogen if the vapor pressure of water at 25 degrees C is 238 mm Hg. A gas exerts a pressure of 600 mmHg at 28C. Once ignited it burns with a pale blue almost invisible flame.
Hydrogen gas oxygen gas and water vapor are collected in eudiometer. C in a total pressure of 732 mm Hg. Total Pressure P H 2 P O 2.
The partial pressure of hydrogen is 7425 mmHg. The levels of water inside and outside the gas-collection bottle are the same. Hydrogen H2 is a colorless odorless gas.
X o x y g e n 03 23. If the entire amount of gas collected is 722 mL what mass of hydrogen gas is collected. What is the barometric pressure at the time the gas is collected.
X o x y g e n 0130. What is the partial pressure of hydrogen gas in a mixture of hydrogen and helium if the total pressure is 600 mmHg and the partial pressure of helium is 439 mmHg. 975 mmHg 760 128289474 atm Result.
The water level in the eudiometer was 1700 mm above the water level in the beaker. A754 b724 c775 d733. The hydrogen gas formed in a chemical reaction is collected over water at 30C at a total pressure of 732 mm Hg.
This is a U-shaped tube containing mercury and connecting the container to the air Figure 2. A volume of 546 mL of hydrogen is collected over water at 30C when the atmospheric pressure is 10045 kPa. Here our free online partial pressure calculator finds the same results but in a fraction of time to save your precious time.
Calculate the partial pressure in mmHg of hydrogen gas. The vapors are lighter than air. P O 2 03.
The partial pressure of Argon is 707 mm Hg. Hydrogen gas produced in the lab by teh reaction of zinc and hydrochloric acid was collected over water at 25degrees C. Because the gas is collected over water it is not pure but is mixed with vapor from the.
The total pressure is 12811 mmHg. MmHg 760 atm Calculations. Latent Heat of Evaporation at boiling point Btulb Jkg 192 447000.
The partial pressure exerted by an individual component is thus proportional to P_Total with the constant of proportionality being n_in_1n_2n_3 the mole fraction.
Solved What Is The Pressure Of Hydrogen Gas Mmhg Chegg Com
Solved Fin Find The Pressure Of Hydrogen Gas Mmhg Chegg Com
Solved How To Find Pressure Of Hydrogen Gas Mmhg Pressure Chegg Com

0 Response to "Pressure of Hydrogen Gas in Mmhg"
Post a Comment